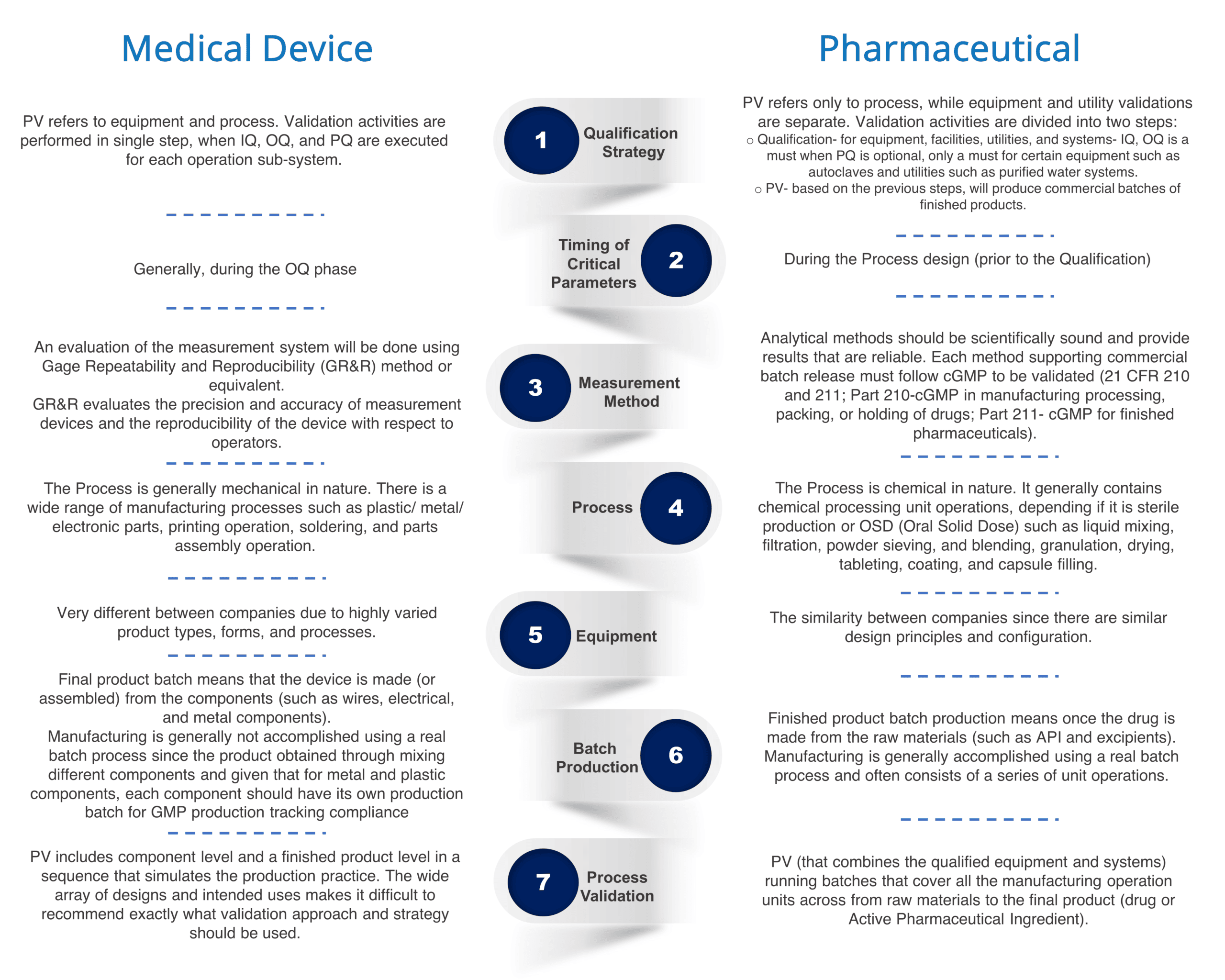
Medical Equipment Validation . Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. The cgmp regulations for validating pharmaceutical (drug). Singapore standards (ss) are nationally recognised documents established by. Guideline on process validation activities. International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. Process verification and process validation are required activities for medical device manufacturers who are required. 10k+ visitors in the past month It is usually done by tests, inspections, and in some.
from rs-ness.com
10k+ visitors in the past month International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Guideline on process validation activities. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Process verification and process validation are required activities for medical device manufacturers who are required. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. The cgmp regulations for validating pharmaceutical (drug). Singapore standards (ss) are nationally recognised documents established by.
Process Validation Pharma vs. Medical Device RS NESS
Medical Equipment Validation Guideline on process validation activities. Singapore standards (ss) are nationally recognised documents established by. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The cgmp regulations for validating pharmaceutical (drug). Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. Guideline on process validation activities. It is usually done by tests, inspections, and in some. 10k+ visitors in the past month International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Process verification and process validation are required activities for medical device manufacturers who are required.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Equipment Validation It is usually done by tests, inspections, and in some. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Singapore standards (ss) are nationally recognised documents established by. 10k+ visitors in the past month. Medical Equipment Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Equipment Validation Process verification and process validation are required activities for medical device manufacturers who are required. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Singapore standards (ss) are nationally recognised documents established by. Guideline on process validation activities. Fda has the authority and responsibility to inspect and evaluate process. Medical Equipment Validation.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Equipment Validation Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Process verification and process validation are required activities for medical device manufacturers who are required. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Singapore standards (ss) are nationally recognised documents established by. It. Medical Equipment Validation.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Equipment Validation The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. The cgmp regulations for validating pharmaceutical (drug). International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Process verification. Medical Equipment Validation.
From templates.rjuuc.edu.np
Equipment Validation Protocol Template Medical Equipment Validation The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. It is usually done. Medical Equipment Validation.
From www.dreamstime.com
Repair and Adjustment of Medical Equipment. Validation of the Ma Stock Medical Equipment Validation The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. The cgmp regulations for validating pharmaceutical (drug). International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Singapore standards (ss) are nationally recognised documents. Medical Equipment Validation.
From www.dreamstime.com
Repair and Adjustment of Medical Equipment. Validation of the Ma Stock Medical Equipment Validation The cgmp regulations for validating pharmaceutical (drug). 10k+ visitors in the past month It is usually done by tests, inspections, and in some. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Guideline on process validation. Medical Equipment Validation.
From aujmsr.com
Validation In pharmaceutical industry Equipment validation A brief Medical Equipment Validation International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. It is usually done by tests, inspections, and in some. Fda has the authority and responsibility to inspect and evaluate process validation performed by. Medical Equipment Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Equipment Validation Guideline on process validation activities. Singapore standards (ss) are nationally recognised documents established by. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Process verification and process validation are required activities. Medical Equipment Validation.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd Issuu Medical Equipment Validation The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The cgmp regulations for validating pharmaceutical (drug).. Medical Equipment Validation.
From www.getreskilled.com
What's a Pharmaceutical Equipment Validation Protocol & Why is it Crucial? Medical Equipment Validation Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Singapore standards (ss) are nationally recognised documents established by. It is usually done by tests, inspections, and in some. Guideline on process validation activities. International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. Fda has the. Medical Equipment Validation.
From www.dreamstime.com
Repair and Adjustment of Medical Equipment. Validation of the Ma Stock Medical Equipment Validation Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Guideline on process validation activities. 10k+ visitors in the past month International regulations for medical devices [1, 2, 3, 4] stipulate validation of manufacturing. The cgmp regulations for validating pharmaceutical (drug). The goal of process validation is to produce a. Medical Equipment Validation.
From ekdoseispelasgos.blogspot.com
Equipment Validation Template Master Template Medical Equipment Validation Singapore standards (ss) are nationally recognised documents established by. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. Guideline on process validation activities. Validation is the process of. Medical Equipment Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Equipment Validation Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. The cgmp regulations for validating pharmaceutical (drug). 10k+ visitors in the past month Fda has the authority and responsibility to inspect and evaluate process validation performed by manufacturers. It is usually done by tests, inspections, and in some. Process verification. Medical Equipment Validation.
From www.dreamstime.com
Repair and Adjustment of Medical Equipment. Validation of the Ma Stock Medical Equipment Validation The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Validation is the process of making sure that you have objective evidence that user needs and intended uses are met. Guideline on process validation activities. The cgmp regulations for validating pharmaceutical (drug). Singapore standards (ss) are nationally recognised documents established by. 10k+. Medical Equipment Validation.
From www.dotcompliance.com
QMS 101 Medical Device Validation Dot Compliance Medical Equipment Validation Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. Singapore standards (ss) are nationally recognised documents established by. Fda has the authority and responsibility to inspect and evaluate. Medical Equipment Validation.
From www.qualitymeddev.com
Process Validation for Medical Devices Overview of FDA Requirements Medical Equipment Validation Clinical evaluation is the assessment and analysis of clinical data pertaining to a medical device in order to verify the clinical safety and. It is usually done by tests, inspections, and in some. 10k+ visitors in the past month Process verification and process validation are required activities for medical device manufacturers who are required. Validation is the process of making. Medical Equipment Validation.
From iziel.com
Process Validation for Medical Devices Process Validation Services Medical Equipment Validation Process verification and process validation are required activities for medical device manufacturers who are required. The goal of process validation is to produce a stable medical device manufacturing process that offers consistent performance. It is usually done by tests, inspections, and in some. Guideline on process validation activities. Validation is the process of making sure that you have objective evidence. Medical Equipment Validation.